Cell Membrane Receptors
Authors
INTRODUCTION
In the early 1900s, while investigating the actions of nicotine and curare, Langley postulated that chemicals act in living cells by way of receptive substances.1 The concept of receptors as molecules that specifically recognize and bind chemical compounds, leading to a series of cellular events culminating in biologic responses, was further developed by pharmacologists and then by endocrinologists. The concept of cellular receptors for chemicals within living tissues was made possible by the availability of highly purified peptide hormones, the development of methods to iodinate them to high specific activity without the loss of biologic activity, and the application of the principle of competitive binding between labeled and unlabeled ligands. Initial evidence for hormones acting through cell surface receptors came from studies using antibodies to peptide hormones that caused cross-linking of hormone-receptor complexes, preventing mobility in the membrane and internalization, thereby blocking or reversing the biologic effects of these hormones.2, 3 In contrast, antibodies to steroid hormones could not achieve the same effects, suggesting that steroid hormone receptors were not located on the cell surface.2 This initial evidence is not quite accurate; there have since been numerous studies demonstrating that functional cell membrane receptors are also present in intracellular organelles and that steroid hormone receptors are present on the cell surface.
The concept of second messengers mediating the effects of peptide hormones was prompted by data from Sutherland in the 1960s showing that adenylate cyclase, an enzyme that forms cyclic adenosine monophosphate (cAMP) from adenosine triphosphate (ATP), was present in cell membranes and that some peptide hormones increase its formation.4 In the ensuing years, there has been a virtual explosion of information on cell membrane receptors and on the signal transducers and effectors that mediate hormone action in the cell.
The binding of peptide hormones, growth factors, cytokines, or eicosanoids to cell membrane receptors activates one or more signal transduction systems, which initiate different cascades of events that alter the concentration of intracellular second messengers, such as cAMP or Ca2+. These signals are subsequently conveyed to the cytoplasm and then to the nucleus through a combination of second-messenger molecules, kinase/phosphorylation cascades, and transcription factor translocation to effect changes in gene expression. Most cell membrane receptors can be grouped into three major classes on the basis of the transducer and effector systems that mediate their action in the cell5 (Table 1). The G protein-coupled receptor (GPCR) family, the largest group of membrane receptors, uses guanine nucleotide-binding proteins (G proteins) to couple to specific intracellular effector systems. The catalytic receptor family is characterized by having effector or enzymatic activity intrinsic to the receptor protein. The channel-linked receptors function as ligand-gated ion channels. Not all membrane hormone receptors fall neatly into one of these three major classes. Receptors for some hormones operate through effector systems that have yet to be definitively established, and others appear to use multiple regulatory pathways.
Table 1. Hormone receptors and their effector systems
G protein-coupled receptors | Catalytic receptors* | Channel-linked receptors* |
Adenylate Cyclase (nicotinic) | Tyrosine Kinase | Acetylcholine |
Luteinizing hormone (LH)/human chorionic gonadotropin (hCG) | Insulin | (γ-aminobutyric acid) |
Insulin-like growth factor 1/somatomedin C | Glycine | |
Follicle-stimulating hormone (FSH) | Epidermal growth factor/transforming growth factor-α |
|
Glutamate/N-methyl-D-aspartate |
|
|
Thyrotropin (TSH) |
|
|
Corticotropin | Colony-stimulating factor-1 |
|
β-Adrenergic catecholamines (β1 , β2) | Fibroblast growth factor |
|
α2-Adrenergic catecholamines (inhibition)
| Platelet-derived growth factor |
|
Interleukins-2, 3, 4, 5, 6, 7 |
| |
Acetylcholine (muscarinic M2, M4; inhibition)
| Growth hormone |
|
Prolactin |
| |
Dopamine 1,2 | Placental lactogen |
|
Serotonin | Erythropoietin |
|
Melatonin (inhibition?) | Nerve growth factor |
|
Glucagon | Serine kinase |
|
Somatostatin (inhibition) | Activin |
|
Growth hormone-releasing hormone | Inhibin |
|
Vasoactive intestinal peptide | Transforming growth factor-β |
|
Endogenous opiate peptides (inhibition) |
|
|
Gastrin-releasing peptide (GRP) |
|
|
Galanin (inhibition) |
|
|
Secretin |
|
|
Calcitonin |
|
|
Parathyroid hormone (PTH) |
|
|
Vasopressin |
|
|
Melanocyte-stimulating hormone |
|
|
Angiotensin II (inhibition/stimulation) |
|
|
Prostaglandins (PGE1, PGE2) |
|
|
A2 Adenosine |
|
|
Guanylate Cyclase |
|
|
Atrial natriuretic peptide |
|
|
Brain natriuretic peptide |
|
|
Phospholipase C/phosphoinositol turnover and calcium flux |
| |
LH/hCG |
|
|
TSH |
|
|
Luteinizing hormone-releasing hormone (LHRH) |
|
|
Thyrotropin-releasing hormone (TRH) |
|
|
Corticotropin-releasing hormone |
|
|
Cholecystokinin B/gastrin |
|
|
Dopamine 1,2 |
|
|
Serotonin |
|
|
Acetylcholine (muscarinic M1, M3, M5) |
|
|
α-Adrenergic catecholamines (stimulation) |
|
|
Angiotensin II |
|
|
Thrombin |
|
|
Bombesin/GRP |
|
|
Calcitonin |
|
|
PTH |
|
|
Vasopressin |
|
|
Oxytocin |
|
|
Interleukin (IL)-1 and 8 |
|
|
Prostaglandin F2α |
|
|
Thromboxane A2/prostaglandin H2 |
|
|
Leukotrienes |
|
|
*May also require G proteins for signal transduction.
Adapted from Kahn CR, Smith RJ, Chin WW: Mechanism of action of hormones that act at the cell surface. In Wilson JD, Foster DW (eds): Williams Textbook of Endocrinology. 8th ed. Philadelphia: WB Saunders, 1992.
Within each class of membrane receptor, there is considerable structural and functional homology, but sufficient differences exist to ensure specificity and diversity of cellular effects. A wide variety of molecules, including plant and microbial toxins, enzymes, viruses, and sperm, use cell membrane receptors to regulate cells. Their receptors may not fall into families described for hormone receptors, but molecules appear to use many of the same mechanisms. Although all cell membrane receptors are proteins, hormonal and nonhormonal ligands vary widely in chemical nature, charge, size, and conformation.
The emergence of new molecular biology techniques in the past few years has permitted cloning of many receptor genes and identification of structural similarities of some receptor proteins to certain oncogene proteins. A family of novel “orphan” membrane receptors has been described that lacks known ligands.6
This chapter provides a general discussion of cell membrane receptors regarding their structure and function, methods of characterization and measurement, and clinical relevance. Various effector systems that mediate hormonal effects on the cell are described, with particular emphasis on glycoprotein hormones of relevance to researchers and clinicians in the field of reproductive biology. Specialized review articles offer more detailed accounts of the topics covered here.7, 8, 9, 10 The mechanism of action of steroid and thyroid hormones has been discussed in Chapter 4.11
MACROMOLECULAR NATURE OF MEMBRANE RECEPTORS
Despite binding a wide variety of water-soluble and some lipid-soluble signaling molecules,2 cell membrane receptors share a number of common structural and functional features. Membrane associated hormone receptors have multiple functional domains that enable them to bind their specific ligands with high specificity and high affinity; to interact with effector systems, directly (e.g. ligand-gated ion channels) or indirectly (e.g. through G proteins); to possess intrinsic enzyme activity (e.g. tyrosine kinases); and to regulate membrane localization or internalization.2 The amino terminus of receptor proteins is extracellular, contains the ligand-binding domain12, 13 and immunologic recognition sites, and may be extensively modified by glycosylation, addition of sulfate or phosphate groups, or disulfide linkages.2 The central region of the receptor consists of one or more transmembrane-spanning domains that anchor the receptor to the membrane and play important roles in the signal transduction process and, in some cases, interaction with ligands.2 The carboxyl terminus, the intracellular region of the receptor, is involved in interactions with effector systems (e.g. G proteins)14 or functions directly as an effector (e.g. tyrosine kinases). This region may also play a role in receptor internalization.2
CELL MEMBRANE RECEPTOR FAMILIES
G Protein-Coupled Receptor Family
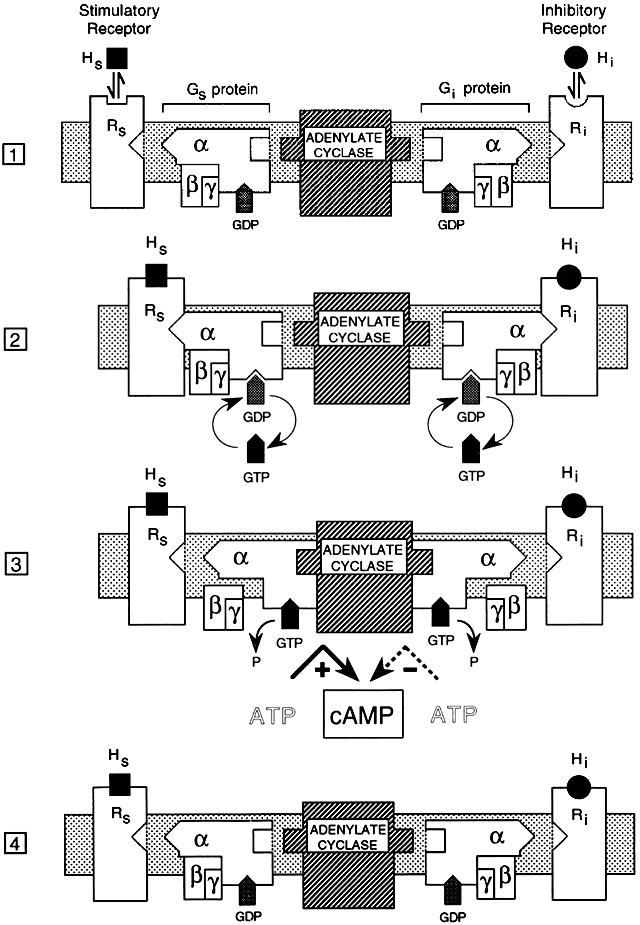
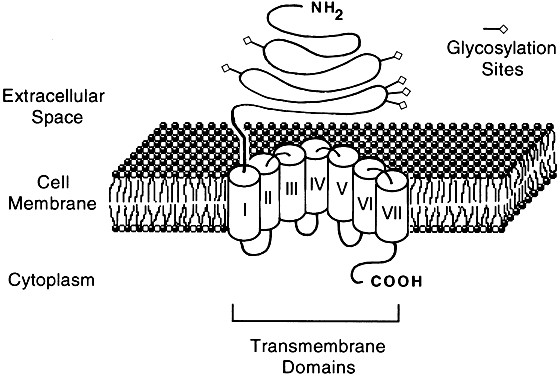
Molecular cloning studies and more traditional biochemical and pharmacologic approaches have revealed a large superfamily of seven transmembrane-spanning receptor proteins that exhibit considerable overlap in their structural features and biologic activities.2, 15, 16, 17, 18, 19 The receptors bind to G proteins that couple them to a variety of effectors that mediate target cell responses20 (Figs. 1 and 2).
The GPCR superfamily can be divided into three groups on the basis of structure:
- Group I, composed of receptors that contain 300–400 amino acids, has a short N-terminal extracellular region, and binds small ligands (<2 kd), such as hypothalamic-releasing hormone and α- and β-adrenergic agents.
- Group II (Fig. 2), which shares considerable homology to group I in the transmembrane region, is composed of receptors that are 700–800 amino acids long, contains a large extracellular domain, and binds large ligands (30–40 kd), such as the glycoprotein hormones.
- Group III receptors, which share very little homology within the seven transmembrane regions with the other two groups, are 400–600 amino acids long, have a large extracellular domain, and bind ligands of intermediate size (4–10 kd), such as calcitonin and vasoactive intestinal peptide.21
G proteins that couple hormone receptors to effector systems are only one class of a large group of intracellular guanosine triphosphate (GTP)-binding regulatory proteins. There are more than 1000 members of the GPCR family.22 GPCRs in this class are derived from at least 16 different genes,20 contain α, β, and γ subunits, and have high molecular weights.23 This group of G proteins can endow the hormone receptor with stimulatory (Gs) or inhibitory (Gi) effects on intracellular second messengers, depending on the type of α subunit contained within the G proteins. Gs proteins activate adenylyl cyclase, and Gi proteins inhibit adenylyl cyclase.18 Certain ligands have stimulatory and inhibitory receptor forms, most notably adrenergic receptors, which can activate (β-adrenergic) or inhibit (α2-adrenergic) adenylate cyclase.5
All signal-transducing GTP-binding proteins operate as molecular switches by sequentially binding and hydrolyzing GTP.23 For the detailed mechanism, see Fig. 1. Binding of a low-molecular-weight agonist to sites within the hydrophobic core formed by transmembrane helices of the receptor induces dimerization and activation by inducing conformational changes in the receptor protein.24 Once activated by G protein binding, adenylate cyclase catalyzes the formation of cAMP from ATP, which leads to activation of protein kinase A, which phosphorylates other cellular proteins, initiating a cascade of other cellular events.
The α subunit has multiple functional domains that are involved in receptor binding, GTP binding, GTP hydrolysis, α- and β-subunit association, and effector interaction and regulation.25 The βγ complex has been proposed to play a structural role in anchoring the G protein to the cell membrane.26, 27 Data indicate that the βγ dimer may also play an important functional role in signal transduction.28 G protein regulation of second messenger activity is complex, exhibiting multiple mechanisms and considerable tissue and receptor specificity. Because each of the subunits exists in multiple forms, there are many possible combinations of the three subunits that comprise the whole G protein complex, each with potentially different activities.
Although G proteins were first described in their role as regulators of adenylate cyclase activity, they also modulate the activity of other second messenger systems and ion channels. Each G protein has unique binding kinetics and affinities for various constituents of the signal transduction cycle.29, 30, 31 The slow intrinsic rate of GTP hydrolysis by Gα proteins is regulated by interactions with GTPase-activating proteins (GAPs).32 There is a newly discovered family of at least 19 mammalian genes for GAPs known as regulators of G protein signaling (RGS) proteins.32 These proteins interact with various cellular constituents involved in feedback regulation of G protein function and coordination of G protein-regulated signaling systems.32
The mechanisms by which GPCRs lead to cell proliferation is beginning to be understood. In brief, GPCRs and tyrosine kinase receptors activate RAS, thereby initiating a cascade of events leading to activation of mitogen-activated protein (MAP) kinases.33 MAP kinases are critical components of growth-promoting pathways and act by phosphorylating key enzymes and nuclear proteins that ultimately regulate the expression of genes essential for proliferation.22 A novel family of enzymes closely related to MAP kinase, designated JUN kinases (JNKs), selectively regulate the activity of the JUN protein, which is a leucine-zipper family transcription factor.22
Glycoprotein Hormone Receptor Subfamily
The gonadotropins, luteinizing hormone (LH), human chorionic gonadotropin (hCG), follicle stimulating hormone (FSH), and thyrotropin (TSH) are composed of an identical α subunit and a unique β subunit. LH and hCG have a high sequence homology in the β subunit and share a common receptor protein. All three receptors have been cloned and have an overall 55% sequence homology.34 In addition to having seven transmembrane domains, all glycoprotein hormone receptors have a large extracellular N-terminal region and a short cytoplasmic C-terminal domain (Fig. 2). The only region in the glycoprotein hormone receptors that is homologous with other GPCRs is the transmembrane domain.35 The large extracellular domain in the LH/hCG receptor is necessary and sufficient for binding hCG and LH.36
Catalytic Receptor Family
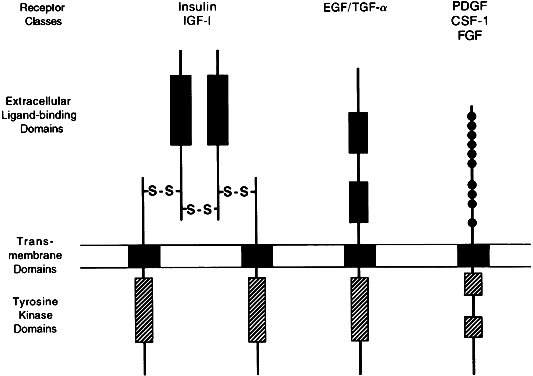
Effector activity is an intrinsic part of the receptor peptide structure for the catalytic receptor family, a group of cell membrane receptors (Fig. 3). Once activated by ligands,5 these single-pass transmembrane proteins act directly as phosphorylating enzymes, mostly through a cytoplasmic domain that functions as a tyrosine-specific protein kinase. Some hormone receptors, such as those for transforming growth factor-β (TGF-β) and for the gonadal peptides, activin and inhibin, act as serine-specific kinases. Threonine-specific receptor kinases have also been described.2
Molecular cloning studies have described three different classes of receptors that contain tyrosine-kinase activity. The most complex class includes receptors for insulin and insulin-like growth factor-1 (IGF-1), with two α and two β subunits, forming two transmembrane domains connected extracellularly by disulfide bridges.37 The second class, represented by the epidermal growth factor (EGF)/TGF-αreceptor, contains an extracellular growth factor-binding domain connected to a cytoplasmic tyrosine kinase domain by a single transmembrane region.38 The third class includes receptors for platelet derived growth factor, fibroblast growth factor, and colony-stimulating factor-1, which contain a single transmembrane domain and a cysteine-rich extracellular domain.39, 40
Autophosphorylation of receptors in tyrosine, serine, or threonine residues by the kinase portion of receptor protein or receptor phosphorylation by other cellular kinases is thought to be an important mechanism of receptor activation,41, 42, 43 possibly by affecting the tertiary structure of the receptor protein.37 Autophosphorylation is also an effective positive feedback mechanism, providing signal amplification. Cross-phosphorylation of receptors for other hormones, such as the phosphorylation of IGF-1 receptors by insulin receptors, may contribute to the diversity of effects of these hormones in target cells.44
Although only a few of the specific protein substrates for receptor tyrosine kinases have been identified, some phosphoproteins are common substrates to several receptors, suggesting another mechanism for overlap in cellular activities of certain hormones.45 In addition to tyrosine phosphorylation, insulin, EGF, and other growth factors appear to regulate cell function through control of other second messenger systems. For example, phosphoinositol turnover ultimately results in activation of protein kinase C and changes in Ca2+ flux46 (Table 1). In the case of EGF, there is evidence for cAMP playing a second messenger role. IGF-1 was reported to activate a nuclear phosphoinositide signaling system that is entirely separate from membrane-bound receptor signaling systems.47
Cloning and characterization of the growth hormone48 and prolactin49 receptors revealed that both are closely related single-pass transmembrane proteins, with structural similarities to cell transport molecules (transferrin) and some homology with erythropoietin, CSF-1, and several interleukins.49 Receptors for growth hormone and prolactin have also been reported to contain or be associated with tyrosine kinase activity, although it is unclear whether tyrosine kinase is an intrinsic property of the receptor molecule.50 Prolactin and growth hormone stimulate protein kinase C activity in vitro51 and appear to phosphorylate the same high-molecular-weight protein.
Channel-Linked Receptor Family
Hormone receptors in this group function as ligand-gated ion channels and belong to a family of homologous, multipass transmembrane proteins (Fig. 4). Channel-linked receptors regulate the flux of calcium, potassium, chloride, and sodium ions across the cell membrane. Ion channels allow movement of ions at very rapid rates (107–109 ions per second) across membranes, several orders of magnitude higher than most active transport mechanisms.52 Ion channel-linked receptors are involved predominantly in rapid chemical synaptic neurotransmission between electrically excitable cells and may also provide a mechanism for autocrine or modulatory effects of certain hormones and neurotransmitters.7 The best understood ligand-gated ion channel is the directly channel-coupled nicotinic cholinergic receptor, consisting of five glycosylated subunits, encoded by separate genes, with each subunit containing four transmembrane-spanning domains53 (Fig. 4). Two major types of ion channel modulation involve indirect, longer-duration mechanisms: phosphorylation by protein kinases and interaction with G proteins. Cyclic AMP-dependent kinases and other kinases phosphorylate the nicotinic acetylcholine receptor, affecting the rate of desensitization and modulating ion channel activity.54, 55 Noncovalent interactions between G proteins and membrane ion channel proteins, such as the muscarinic acetylcholine-regulated potassium channel and the β-adrenergic-gated calcium channel,56 also appear to play an important role in ion channel gating.2, 57
EFFECTOR SYSTEMS FOR MEMBRANE RECEPTORS
The major effector systems used by peptide hormones and neurotransmitters operate through mechanisms that involve a series of protein phosphorylation steps or that involve the direct effect of ions on cell function. Generally, GPCRs and catalytic receptor kinases act through phosphorylation-mediated processes. Ion-induced changes regulated by channel-linked receptors can involve phosphorylation of protein kinases and the direct effects of ions.
Adenylate Cyclase
Cyclic AMP, the prototypical second messenger, is found in all eukaryotic cells. It is synthesized from ATP by the plasma membrane-bound adenylate cyclase and hydrolyzed by cAMP phosphodiesterases to form adenosine 5'-monophosphate (5'-AMP). This continuous cycle of formation and hydrolysis forms the basis of many cellular activities. GPCRs exert their effect on cAMP levels through activation of various G proteins that bind GTP and activate or inhibit adenylate cyclase (Fig. 1). Altered cellular concentration of cAMP causes changes in the level of regulated proteins that the enzyme phosphorylates, resulting in changes in metabolic activity, including effects on gene expression.
Guanylate Cyclase
Some hormones, such as atrial natriuretic peptide, have been shown to affect the activity of guanylate cyclase, an enzyme found in soluble and membrane-associated forms. Guanylate cyclase catalyzes the formation of cGMP from GTP, which activates protein kinase G.58 Whether membrane-associated guanylate cyclase is an intrinsic part of the receptor for atrial natriuretic peptide, directly serving receptor and effector functions, remains to be resolved.58
Phosphatidylinositol Turnover and Calcium Flux
A number of hormones exert their effects through calcium ions and diacylglycerol as second messengers. Most hormones (e.g. luteinizing hormone-releasing hormone [LHRH], TSH) that stimulate phosphoinositide turnover operate through G proteins coupled to phospholipase C (PLC) activity.59 Receptors containing tyrosine kinase activity may also stimulate phosphoinositol turnover.2
PLC exists in multiple forms, classified as α, β, and γ, each with several subtypes.60 Only PLC-β is sensitive to G protein regulation,61 and subtype PLC-β2 is activated by βγ dimers.62 PLC causes the conversion of membrane-bound phosphatidylinositol 4,5-bisphosphate to inositol 1,4,5-triphosphate (IP3), which acts as a second messenger to release of Ca2+ from intracellular storage compartments.63 In the same reaction, PLC also causes the production of diacylglycerol, which activates various protein kinase C isozymes64 and calcium-dependent kinases, and releases arachidonic acid, which is a precursor to eicosanoids and other signaling agents. Arachidonic acid, through its conversion to several eicosanoids, regulates a number of cell processes through protein kinase C pathways.64 IP3 and Ca2+ interact with specific calcium-dependent kinases that, like protein kinase C, phosphorylate other cellular proteins that result in changes in cell activity. In addition to PLC, there are several other phospholipases (e.g. PLA2, PLD) that may play important, but less well understood, roles in cell signaling through activation of protein kinase C.64
The regulation of intracellular calcium ion concentration plays a critical role in cell signaling. Although extracellular Ca2+ levels are about 10-3 to 10-2 M, intracellular levels are regulated at a steady state concentration of approximately 10-7 M. The steep concentration gradient across the cell membrane is maintained by a variety of ion pumps, channels, and transport molecules. Many peptide hormones and other ligands that bind to cell membrane receptors influence intracellular Ca2+ homeostasis.
Dual or Multiple Regulatory Systems
Activation of dual signaling pathways by a single species of ligand interacting with two different receptor forms has been described for several GPCRs (i.e. stimulatory and inhibitory adrenergic or cholinergic receptors).65 Interest has focused on a single ligand binding to a single receptor that activates parallel second messenger systems,65 such as the activation of cAMP, phosphoinositide turnover, and mobilization of Ca2+ by LH,66 LHRH,67 and α-adrenergic68 receptors. Different signaling pathways may be activated by the α and βγ complexes of a single G protein or by different G proteins with distinct α subunits,28 although the data appear to support the latter mechanism.
The ability of a hormone-receptor complex to activate more than one type of effector system is thought to play an important role in tissue specificity of hormone response. For instance, certain hormones can produce different signals in different types of cells.69
An expanding body of literature suggests that there is a considerable amount of interaction or “crosstalk” between the various effector system pathways used by ligands of all types,65, 70 such as the modulation of estrogen receptor functions by EGF,71 IGF-1,72 and TGF-β73, 74 and enhancement of transcriptional activity of progesterone and estrogen receptors by dopamine.75, 76
Alternate or Novel Modes of Hormonal Regulation
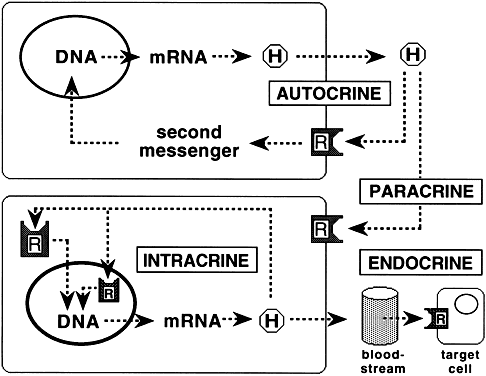
An expanding body of literature indicates that many membrane receptors participate in modes of hormonal regulation in addition to the classic endocrine regulation by circulating hormones (Fig. 5). The regulation of neighboring cell function by hormones released into the extracellular space is known as paracrine regulation. Juxtracrine regulation is a form of paracrine regulation in which the ligand translocates to the cell surface to interact with its cognate receptor on the surface of an adjacent cell. Hormone binding to surface receptors of cell of origin is known as autocrine regulation. A newly demonstrated form of autocrine regulation, called intracrine regulation, consists of control of cellular activity by unsecreted hormones through binding to intracellular receptors. The hormone hCG has been shown to exhibit each of the described forms of regulation: endocrine regulation of progesterone production by corpus luteum, paracrine regulation between syncytiotrophoblasts and cytotrophoblasts,77 autocrine regulation,78 and intracrine regulation of hCG biosynthesis in choriocarcinoma cells.79
Multiple levels of regulation are not unique to cell membrane receptors. Members of the steroid and thyroid hormone receptor superfamily also exhibit similar regulatory mechanisms. One example is progesterone produced by corpus luteum, which acts on a number of distant tissues and acts locally within the corpus luteum through paracrine and intracrine mechanisms.80
NUCLEAR RECEPTORS FOR PEPTIDE HORMONES AND RELATED LIGANDS
Contrary to the popular belief that receptors for peptide hormones, growth factors, cytokines, and eicosanoids are present only on the cell surface, many studies have also demonstrated their presence in nuclei and several other intracellular organelles that are associated with protein synthesis and breakdown 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126 (Table 2). The receptors in the cytoplasmic organelles could be those in biosynthetic and catabolic routes. However, receptors in nuclei cannot be there because of these reasons. Nuclear receptors are similar but may not be totally identical to those on the cell surface. They have been found in nuclear membranes, chromatin, nuclear matrix, and nucleolus. Each of these sites has specific roles, and the presence of receptors suggests that treatment of isolated nuclei can regulate the transcription of target genes and processing and export of RNA into the cytoplasm, which has turned out to be true.
Table 2. Nuclear receptors for peptide hormones, growth factors, cytokines, and eicosanoids
Receptor | Function* |
Luteinizing hormone/human chorionic gonadotropin | Increase NTPase activity and chromatin solubility |
Epidermal growth factor/transforming growth factor-α | Inhibit LH/hCG receptor gene transcription, increase receptor autophosphorylation, activation of MAP kinase, and increase in RNA transport |
Fibroblast growth factor increase | Stimulate phosphorylation of nucleolin, phosphoglycerate (PgK)1 activity and decrease the activity of Pg2, and increase the transcription of ribosomal genes |
Platelet-derived growth factor |
|
Nerve growth factor |
|
Insulin | Increase NTPase activity and mRNA transport |
Insulin-like growth factor-1 | Activation of phosphoinositide signaling pathway |
Prolactin | Activate protein kinase C signaling pathway |
Growth hormone |
|
Angiotensin II |
|
Prostaglandin E2 | Increase intranuclear Ca2+ levels and transcription of FOS gene |
Prostaglandin F2α |
|
Vasoactive intestinal peptide |
|
Tumor necrosis factor-α |
|
Interferons |
|
Interleukins |
|
Endothelin |
|
*The functions of many nuclear receptors have not been determined. However, their location indicates that they are involved in the regulation of target gene transcription and processing and transport of RNA into the cytoplasm.
The presence of nuclear receptors raises many questions. How do receptors get into the nucleus? How does the ligand reach nuclear receptors? How important are nuclear receptors compared with those in cell surfaces? What type of signaling pathway does the nuclear receptor use? Although definitive answers are not available, deductive reasoning may help in better understanding nuclear receptors.
The source of nuclear receptors in part could be endocytosed cell surface receptors that escaped the degradative route and reached the nucleus by an unknown mechanism. It is also possible that some nuclear receptors have never been on the cell surface. Instead, they could be directed to the nucleus after having been synthesized in rough endoplasmic reticulum and posttranslationally modified in the Golgi complex. Many nuclear receptors do not have a classic nuclear localization signal sequence, suggesting they may contain other sequences that serve the same purpose, or that they piggyback on other nuclear proteins.
The ligand may come from the dissociation of endocytosed receptor-ligand complexes. In many cases, cells can make a ligand and its receptors. In such a case, there is no point for the ligand or its receptors to leave the cell to come back in; both may be directed to the nucleus.
Cells do release ligands to the extracellular environment. These ligands may bind to the same cell surface receptors to mediate a set of actions that only plasma membrane receptors could mediate. The externalized ligand may also bind to surface receptors of adjacent cells that do not make ligand but contain its receptors. Ligands that bind to these cell surfaces and to nuclear receptors may come from the extracellular environment.
It is difficult to determine the relative importance of nuclear versus cell surface receptors as long as both are operating in the cell. The simplest view is that both receptors are important and necessary to mediate diverse actions of ligand. Nuclear receptors may assume additional importance in case of growth factors that are involved in cancer.
Signaling pathways used by nuclear receptors appear to be similar to those used by cell surface receptors. Nuclei contain their own signaling pathways. In some cases, liganded or free nuclear receptors may bind DNA sequences to regulate the transcription of target genes.
REGULATION OF CELL MEMBRANE RECEPTORS
Biosynthesis and Catabolism of Cell Membrane Receptors
Cell membrane receptors, like all other membrane proteins, are synthesized in rough endoplasmic reticulum, posttranslationally modified in the Golgi complex, and then inserted into the plasma membrane as mature functional receptors that are freely mobile laterally within the lipid bilayer. Some of the newly synthesized receptors may be diverted to other cellular membranes and even to nuclei. Hormone binding causes most membrane receptors (e.g. insulin, EGF, TSH, LHRH) to aggregate on the cell surface, forming dimers or multimers that are eventually internalized through clathrin-coated pits.127 Microaggregation of receptor proteins is thought to play a role in signal transduction, as evidenced by the mimicry of hormone action by unliganded receptors cross-linked by bivalent antibodies.128
Receptor proteins are in a constant state of turnover, with their synthesis and degradation regulated by a variety of factors, most notably by the ligand itself. After the hormone-receptor complex has become internalized, some receptor proteins become dissociated from the ligand and may be recycled as many as 50 times before degradation,129, 130, 131 whereas others are destroyed by lysosomes after a single pass. Internalization is also a way to introduce the ligand and receptor to the cytoplasm, where they are potentially free to interact with other cellular or nuclear factors. Chemical modification, such as phosphorylation, can affect the half-life of receptor proteins, changing the steady-state balance between synthesis and degradation.
In addition to the internalization of membrane receptor proteins into the cell of origin (i.e. endocytosis), receptors are recycled back to the cell surface (i.e. retroendocytosis) and act as carriers for the transport of hormone through cells (i.e. transendocytosis or transcytosis).
Upregulation and Downregulation of Receptors
Changes in hormone binding to tissues are usually the result of changes in the numbers but not affinity of receptors. The hormone binding and response decrease with repeated exposure to hormone in downregulation (i.e. desensitization or refractoriness), whereas in upregulation, the reverse happens. In downregulation, the decrease of hormone binding and hormone response depends on hormone concentration and duration of treatment, is reversible, and is seen in vitro and in vivo. The following mechanisms have been suggested for receptor desensitization or downregulation: internalization or compartmentalization of the receptor,132 enhanced receptor degradation, decreased receptor synthesis, uncoupling of the receptor from effector system protein, and a decrease in activities downstream from effector systems.133 In some cases, the end product of receptor activation negatively feedback on receptor mRNA levels. For example, in the case of LH/hCG receptors, mevalonate kinase, an enzyme involved in cholesterol biosynthesis, and its two other interacting protein partners, suppress LH/hCG receptor mRNA translation and promote its degradation.134 Changes in responsiveness can be modulated by the hormone itself or by other hormones or second messengers.135 Activation of protein kinase may be involved in hormone-induced upregulation and downregulation.133, 136, 137, 138 Some hormones, such as prolactin, may act as positive regulators of their own receptors (i.e. upregulation).139 More commonly, hormones act as positive regulators of receptors for other hormones. Well-known examples of heterologous positive regulation are the stimulation of LH receptors by FSH in the ovarian follicle140 and by prolactin in Leydig cells.141 Negative heterologous regulation has also been identified, as in hormones that act through stimulation of protein kinase C, but it is less well documented.142 The phenomenon of downregulation is seen more often than upregulation and has practical relevance. For example, to obtain optimal therapeutic benefit, the intervals of hormone administration need to be spaced to avoid the downregulation period.
Spare Receptors
The concept of spare receptors came from data that showed that maximal hormonal response is seen with occupancy of only a fraction of the total number of available receptors.2 Although this may be true for a given response, occupancy of additional receptors may be needed for other actions of the hormone. The pool of unoccupied receptors may play an important role in signal amplification and to control the duration of response.
GENOMIC EFFECTS OF PEPTIDE HORMONES
The current model of peptide hormone regulation of gene expression entails the interaction of a hormone with its membrane receptor and resultant activation of second messengers (e.g. cAMP). Cyclic AMP activates protein kinase A that phosphorylates transcription factors, such as cAMP response element-binding protein (CREB) and CCAAT/enhancer-binding proteins (C/EBPs),142 which bind to specific DNA sequences in the regulatory regions of target genes. Activated protein kinase A can also phosphorylate coactivator CREB-binding protein (CBP), which interacts with CREB143 and nuclear receptors.144 CREB and CBP also interact with the AP-1 transcription factor complex, composed of two JUN or two FOS proteins or a FOS and JUN heterodimer, which bind to the AP-1 regulatory site on protein kinase C-regulated genes, leading to coordinate regulation of gene expression.145 Internalized receptor or hormone may also reach the nucleus and regulate transcription or expression of certain genes.146, 147, 148 An “insulin response element” was identified in the promoter region of the prolactin gene that was thought to be activated by binding to a nuclear form of the insulin receptor.149 Other studies show that insulin acts at the gene transcription level by activating AP-1,150 activation of a newly described DNA-binding protein called glucose response element-binding protein,151 activation of C/EBP,142 and induction of HIF-1α/aryl hydrocarbon receptor nuclear translocator (ARNT).152 Peptide hormones may also regulate gene expression indirectly by controlling the nucleocytoplasmic transport of mRNA, affecting translational efficiency, half-life or stability of mRNA, or processing and degradation of translated proteins.2
A number of extracellular signaling polypeptides interact with specific cell membrane receptors that trigger activation of latent cytoplasmic transcription factors called signal transducers and activators of transcription (STATs) by triggering phosphorylation on tyrosine.153 Specificity is achieved through various mechanisms, including the observation that multiple receptors that activate the same STAT are usually not expressed on the same cell.153 Targeted disruptions of mouse STAT genes result in particular phenotypes; for example, disruption of STAT5A154 or STAT5B155 results in the lack of mammary gland development and lactation.
CELL MEMBRANE RECEPTORS IN PATHOLOGIC STATES
The absence of cell membrane receptors, functional or structural defects in receptor proteins, and the presence of humoral receptor antibodies account for a number of pathologic conditions.2 Type II diabetes mellitus156 is a common example of hormone resistance, resulting from decreased insulin receptor number and genetic defects in the receptor or action pathways. Parathyroid hormone resistance occurs because of defects in the Gs protein that couples the receptor to adenylate cyclase.157 Production of autoantibodies to peptide hormone receptors forms the basis of several endocrine diseases, such as type I diabetes mellitus (i.e. insulin receptor158), myasthenia gravis (i.e. acetylcholine receptor), and some cases of premature ovarian failure (i.e. FSH or LH receptors). TSH receptor antibodies in Graves' disease159, 160 bind to TSH receptors and mimic the hormone, resulting in overstimulation of thyroid hormone secretion.
Receptor overproduction may play an important role in cell proliferation during cancer. Certain oncogene products, such as ERBB, which is highly homologous to the EGF receptor, may function as growth factor receptors (i.e. tyrosine-specific kinases). These oncogene products lack a functional hormone-binding domain, resulting in constitutive activation of second messengers that leads to uncontrolled cell growth and division.161 Some oncogenes encode proteins that mimic components of the receptor (e.g. SRC) or G protein complex (e.g. RAS) or second messenger systems in peptide hormone action pathways.162, 163 Still other oncogene products, such as FOS and JUN, act as transcription regulatory proteins that bind to specific, hormone-dependent enhancer regions (i.e. AP-1 sites) of regulatory DNA on target genes.164
Persistently high concentrations of hormone can result in “specificity spillover,” when the hormone in abundance interacts with receptors for other related hormones to induce uncharacteristic biologic effects.2 In acromegaly, growth hormone overproduction leads to a syndrome of systemic effects in part caused by growth hormone binding to prolactin receptors.165 Similarly, in trophoblastic disease, high circulating levels of hCG can cause hyperthyroidism because of binding of hCG to TSH receptors.166
Normal receptor activity can be restored in receptor-defective mammalian cells by facilitating formation of intercellular junctional communication with normal cells. The identification of membrane receptor genes makes it ultimately possible for gene transfer, as has been accomplished for nerve growth factor receptors. Such knowledge may help devise strategies to block inappropriate expression of receptors in some endocrine tumors, such as some adrenal tumors that contain receptors for gonadotropins, TSH, epinephrine, and norepinephrine.
The discovery of activating mutations in GPCRs and G proteins in several disease states, including cancer, supports a role for GPCRs in normal cell growth.167 Constitutively active TSH receptor mutations have been found in 30% of thyroid adenomas. Activating mutations in various GPCRs for neuropeptides and prostaglandins have been implicated in a variety of human cancers, including small cell lung carcinoma, colon adenomas and carcinomas, and gastric hyperplasia and cancer. Functional GPCRs have been found in the genome of Kaposi sarcoma-associated herpesvirus,168 and the evidence suggests that these viral GPCRs are sufficient to subvert normal growth control.
Human gene mutations have been described for at least three genes (i.e. GnRH, GnRH receptors, and DAX-1) that cause inherited hypogonadotropic hypogonadism resulting in altered gonadotropin secretion and that affect hypothalamic, pituitary, and gonadal function.169 Activating mutations in the LH receptor result in familial male-limited precocious puberty but have no consequences in females.169, 170, 171 Homozygous or compound heterozygous inactivating mutations in the LH receptor cause Leydig cell hypoplasia, resulting in sex reversal or milder forms of undervirilization in men.171 In females, these inactivating mutations lead to amenorrhea but otherwise normal pubertal development.171
Inactivating mutation of a single amino acid (Ala189 to Val) in FSH receptor has been found in women with pure ovarian dysgenesis characterized by high gonadotropin levels, streaky gonads, and primary amenorrhea.172 Males with the same mutation display various degrees of spermatogenic failure or absolute infertility.172, 173, 174
Mice lacking FSH receptors were generated by targeted gene disruption by homologous recombination.175 Mutant males displayed small testes, partial spermatogenic failure, and reduced fertility. FSH levels were threefold higher in FSH-/- males compared with normal males, whereas females showed a 15-fold decrease in FSH levels. Mutant males showed a 35% decrease in testosterone levels. LH and its receptor levels were normal. The homozygous mutant females had thin uteri and small ovaries and were infertile because of a block in folliculogenesis before antral follicle formation. The mice exhibited enlargement of the anterior lobe of the pituitary, with decreased numbers of FSH-positive cells.
NONGONADAL DISTRIBUTION OF LUTEINIZING HORMONE AND HUMAN CHORIONIC GONADOTROPIN RECEPTORS
Contrary to the widely held popular belief that LH/hCG receptors are only present in gonadal tissues, several studies have demonstrated their presence in a number of nongonadal tissues176, 177, 178, 179, 180, 181 (Table 3). The receptors in nongonadal tissues have been demonstrated at the mRNA, protein, hormone-binding, and functional levels. The nongonadal distribution of LH/hCG receptors is not species specific. They have been found in humans, monkeys, pigs, sheep, cows, rats, rabbits, mice, turkeys and even catfish. Receptor levels in nongonadal tissues are generally lower than in gonadal tissues. Functions regulated by LH and hCG depend on the type of nongonadal tissue (Table 3). These are relevant to normal functions and to a better understanding of benign and malignant diseases in nongonadal target tissues. For example, LH may play an important role in promoting endometrial cancer and inhibiting prostate cancer; similarly, hCG may protect against breast cancer and promote choriocarcinomas.
Table 3. Nongonadal distribution of luteinizing hormone/human chorionic gonadotropin receptors and their putative functions
Tissue | Putative functions* |
Placenta | Regulation of hCG biosynthesis, differentiation of cytotrophoblasts, promote the invasion of intermediate trophoblasts and increase indoleamine 2,3- dioxygenase |
Fetal membranes | Weakening of membranes through increase in cyclooxygenase-1 (COX-1) expression |
Decidua | Increase COX-2 expression and differentiation of stromal cells |
T cells, monocytes, and macrophages | Increase monocyte chemoattractant protein-1 expression |
Umbilical cord | Relax umbilical vessels through increase in prostaglandin2 and decrease in thromboxane A2, and keep the cord supple |
Uterus | Differentiation of stromal cells, increase COX-2 expression in stromal and glandular epithelial cells and maintain myometrial quiescence by downregulating gap junctions and decreasing intracellular Ca2+ levels |
Oviduct | Increase OGP and COX-2, release sperm bound to epithelial cells, and enhance growth of early embryos in cocultures with epithelial cells |
Urinary bladder | Maintain normal functions; chronic elevation of LH may lead to incontinence |
Skin | Regulate androgen metabolism and action |
Adrenal cortex, zona reticularis | Increase dehydroepiandosterone sulfate secretion |
Brain | Neuroendocrine regulation of LH synthesis, behaviors, neurotropic and neurotransmitter functions |
Neural retina | Visual processing of information |
Breast | Promote differentiation of epithelial cells, increase apoptosis and regulate synthesis of macromolecules that are important in preparation for lactation and which also happens to protect against cancer |
Prostate | Regulate androgen metabolism and action and inhibit cell growth that are consistent with protection against cancer |
Epididymis | Sperm transport and maturation |
Seminal vesicles | Secretory functions |
Sperm | Sperm maturation |
*In some cases, the inferences were drawn from the biologic effects of LH and hCG, and in others, the functions are suggested by receptor distribution in nongonadal tissues.
Translational research and intuitive reasoning on the possible clinical benefits of nongonadal action of LH and hCG suggest that hCG may be clinically useful (Table 4). Because hCG is growth inhibitory, it may be useful in the treatment of prostate cancer after patients have been castrated to reduce the level of androgens.
Table 4. Potential therapeutic uses of human chorionic gonadotropin
Increasing pregnancy rates in ART
Prevent miscarriages
Prevent prematurity
Treatment of gynecologic infections, including HIV
Rheumatoid arthritis
Endometriosis
Protection against breast cancer
Kaposi sarcoma
LH and hCG function as hormones, growth factors, and cytokines. Although this broad spectrum of actions is not unique to hCG, it nevertheless raises a question about what might be the phylogenetic significance of such a wide regulation of bodily functions by a reproductive hormone.
MEMBRANE RECEPTORS IN BEHAVIOR
Intraperitoneal or intracerebroventricular injections of highly purified hCG on the morning of proestrus of cycling female rats were reported to change several hippocampus-associated behaviors. The hCG-treated animals were generally less active and showed less exploratory behavior and decreased food neophobia compared with saline injected control animals.182 In another study, hCG administration to rats affected sleep-wake phases and other associated behaviors that can collectively be described as decreased activity.183 These effects were suggested to be mediated by increased prostaglandin D2 and decreasing prostaglandin E2 in areas of the brain that control these activities.183 The implications of behavioral effects of hCG are that the multitude of behavioral changes seen during pregnancy probably are caused by small amounts of peripheral hCG reaching the brain centers that control behaviors, including nausea and vomiting.
METHODS FOR THE STUDY OF MEMBRANE RECEPTORS
There are two requirements for studying membrane receptors for peptide hormones: highly purified hormone must be available, and the hormone must be radiolabeled to as high a specific activity as possible without loss of biologic activity. Competitive binding experiments can then be performed using labeled and unlabeled peptide hormones. The total amount of added hormone and free hormone are quantitated by radioimmunoassay or radioreceptor assay, and the free fraction is subtracted from the total fraction to obtain the quantity of hormone specifically bound to receptors. Although this is a valid approach, it is cumbersome, time consuming, and somewhat inefficient. The most popular method of radiolabeling the peptide hormones is iodination using 125I or 131I. The chloramine T technique of radioiodination is too severe for some hormones such as FSH. For these, the milder lactoperoxidase technique is preferable. These two methods of iodination require the presence of tyrosine residues in molecules. For peptides that do not contain tyrosine, methods are available for attachment of iodinated tyrosine groups to protein molecules by amide bonds. Tritium ([3H])-labeled peptide hormones have also been used, but they are not popular because the labeling procedure is time consuming and yields low specific activity of the [3H]-peptide product. Various types of tissue samples, such as whole organs, tissue sections, intact cells, cell homogenates, crude and highly purified subcellular fractions, and solubilized or highly purified receptor protein molecules, can be used.
The following criteria must be met in defining hormone binding as true receptor binding:
- The binding must be saturable (Fig. 6A), indicating a finite number of sites.
- The binding must be tissue specific, hormone specific, and stereospecific, and must be consistent with the known physiologic effects of the hormone.
- The binding must be of high affinity and in agreement with circulating hormone concentrations.
- The binding must be reversible, consistent with reversal of biologic effects after removal of the hormone.
These criteria generally distinguish specific binding from nonspecific binding measured in the presence of excess unlabeled hormone. The nonspecific binding represents binding to the receptor and to inert organic or inorganic materials that hormones come in contact with during the binding studies, such as glass or plastic surfaces.
Binding studies are conducted to define optimal conditions with respect to time and temperature of incubation, pH of incubation media, buffer composition, and ionic requirements. Most membrane receptor-bound hormones dissociate in a biphasic manner; a rapid initial dissociation phase is followed by a slow dissociation phase that lasts for several hours but does not reach complete dissociation. The nondissociated hormone is intact and can be completely eluted from the receptor by exposure to low pH. The extent of dissociation is inversely related to temperature and length of incubation. The initial association and dissociation rate data are used to calculate rate constants for association and dissociation after the data satisfy the second- and first-order rate equations, respectively.
Using optimal binding conditions, further binding studies are performed, such as dependence of binding on the amount of membrane protein. This binding is usually only linear for a relatively narrow concentration range of membrane protein. Above this linear region, the binding curve plateaus; possible reasons are steric hindrance for hormone binding, and endogenous proteases degrading the hormone at high membrane protein concentrations.
Total, specific, and nonspecific hormone binding depend on the concentration of hormone. This relationship is linear at low hormone concentrations. As increasing concentrations of hormone are added, total and nonspecific binding do not reach saturation, whereas specific binding reaches a plateau, reflecting saturation. Saturation binding experiments can be performed with increasing concentrations of labeled hormone or a fixed low concentration of labeled hormone and increasing concentrations of unlabeled hormone. The latter method is best when the labeled hormone is expensive, or when handling large quantities of radioactive material is to be avoided. Nonspecific binding is determined and subtracted from total binding. Both methods should give identical results.
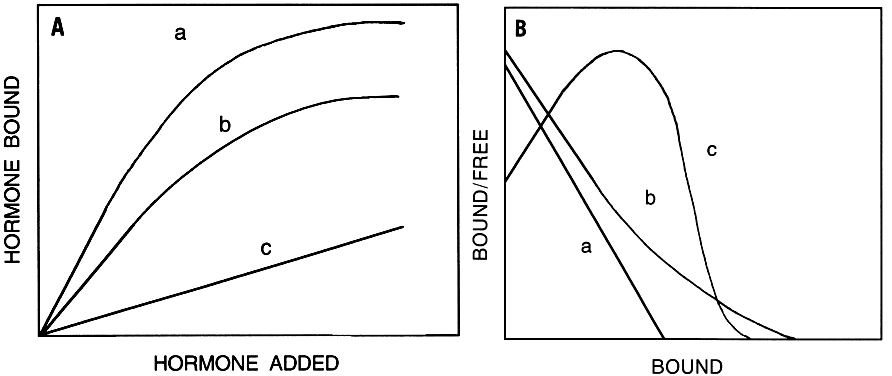
Various methods are used to calculate binding affinity and the capacity of receptors from equilibrium saturation binding data. Scatchard plot analysis184 is the most widely used method. Scatchard originally described this method for the analysis of binding of small molecules to soluble proteins, for which diffusion and other problems that face membrane receptors are nonexistent. Hill analysis is used to determine the Hill coefficient, which is a measure of the cooperativity of ligand-receptor interaction.
All methods of receptor binding analysis require that the hormone molecules are homogeneous, there is no hormone or receptor degradation, hormone receptor binding is completely reversible, and binding saturation is achieved. Analysis of data not meeting these criteria gives inaccurate binding constants. Scatchard plot analysis requires graphing of the data on bound/free hormone as a function of concentration of bound or total hormone. The Scatchard plots can be linear or nonlinear, with upward or downward concavity (Fig. 6B). Linear Scatchard plots indicate the presence of a single homogeneous population of binding sites. Nonlinear Scatchard plots indicate the presence of two or more populations of noninteracting binding sites or a single population of binding sites for which hormone-occupied receptors interact with unoccupied receptors to increase (positive cooperativity) or decrease (negative cooperativity) their affinity. There is an experimental kinetic method to test for the presence of negative cooperativity among receptors. Positive cooperativity among membrane hormone receptors is rarely found. In all cases, the slope of the line is the negative reciprocal of the apparent receptor affinity, and the x-axis intercept gives the apparent total number of available binding sites. The number of sites can be expressed as mass of ligand bound per unit of protein, assuming 1:1 binding of ligand to receptor. The binding capacity can be converted to the number of receptor molecules using Avogadro's number.
Various graphical methods of analysis are available to calculate binding constants. The constants are considered “apparent”, allowing for the possibility that these constants may be different in vivo. The Km of hormone for biologic effect often exceeds the Kd for hormone binding. This discrepancy at least partly results from studying hormone binding and biologic effect under different experimental conditions. Hormone binding and biologic response cannot be experimentally dissociated.
Development of a multitude of effective agonists and antagonists for hormones, as well as for the second messenger systems they activate, has produced a valuable armamentarium for probing the mechanism of action of membrane receptors. The specificity and stereoselectivity of receptors are determined by the ability of increasing concentrations of various related and unrelated unlabeled hormones and different stereoisomers of unlabeled hormones to compete with labeled hormone for binding. The data are expressed as relative affinity values or dose required for 50% inhibition of labeled hormone binding and percent cross-reactivity.
Target tissues contain multiple cell types, and not all cells are targets of the same hormone; therefore, heterogeneity may exist among cell types with respect to receptor distribution. Biochemical receptor studies using tissue homogenates do not reveal this information, and the binding capacity may artifactually be lowered because of the contribution from non-receptor-containing or low-receptor-containing cells. More specific information on individual cells can be ascertained by quantitative light and electron microscope autoradiographic techniques using radiolabeled ligands. Alternatively, direct or indirect immunocytochemical techniques using fluorescence-tagged antireceptor antibodies and antihormone antibodies, respectively, can be used. These techniques will yield qualitative data on the cellular distribution of receptors.
There are two types of cell membrane receptor antibodies: antibodies that bind to the hormone binding site and competitively inhibit hormone binding, and antibodies that bind to a distinct site that does not interfere with hormone binding. Some bivalent receptor antibodies mimic some acute actions of the hormone, in part because of cross-linking and aggregation of the receptors. Membrane receptor antibodies have been used in radioimmunoassay, although receptor measurements are not yet routine diagnostic tools as they are for steroid hormone receptors. Using molecular cloning techniques, the complete amino acid sequences can be readily determined and homologies with other proteins calculated, facilitating detailed structure-activity studies using site-directed mutagenesis and other methods.
CONCLUSIONS
In multicellular organisms, regulation of cell growth and differentiation, metabolic processes, reproductive function, and adaptation to the environment depend on cell-cell communication. Hormones, growth factors, and neurotransmitters form a communication network linking diverse cell types widely distributed throughout the body. The transducer and effector systems used by membrane receptors to relay hormonal signals and alter cellular activities are varied and complex, providing specificity and diversity of response.
Hormones that readily pass through the cell membrane lipid bilayer, such as the steroid and thyroid hormones, exert their effects through binding to nuclear receptors. Peptide hormones, growth factors, and neurotransmitters interact with receptors located in cell membranes. However, this distinction is arbitrary and there is considerable overlap and convergence forming crosstalk between peptide and steroid hormone action.
The use of molecular cloning techniques has greatly enhanced our understanding of the structure and function of membrane receptors and their associated second messenger systems. The demonstration of multiple modes of regulation by hormones, such as paracrine, juxtracrine, autocrine, and intracrine, as well as the complex interactions between hormones at the levels of the receptor or second messenger system, including activation of transcription by diverse families of trans-activating proteins, indicate that regulation of life processes is exquisitely controlled.
Molecular techniques have been used to describe the genetic basis of a number of endocrine diseases related to receptor dysfunction, including mutations, facilitating their detection and treatment. Rapid expansion of our understanding of cell membrane receptors will undoubtedly yield valuable diagnostic and therapeutic modalities for the management of reproductive and endocrine disorders.
REFERENCES
Langley JN: On nerve endings and on special excitable substances. Proc R Soc Lond 78: 170, 1906 |
|
Kahn CR, Smith RJ, Chin WW: Mechanism of action of hormones that act at the cell surface. In Wilson JD, Foster DW, Kronenberg HM, Larsen PR (eds): Williams' Textbook of Endocrinology, pp 95–143. 9th ed. Philadelphia: WB Saunders, 1998 |
|
Pastan I, Roth J, Macchia V: Binding of hormone to tissue: the first step in polypeptide hormone action. Proc Natl Acad Sci USA 56: 1802– 1809, 1966 |
|
Sutherland EW: Studies on the mechanism of hormone action. Science 177: 401, 1972 |
|
Alberts B, Bray D, Lewis J et al: Cell signaling. In Molecular Biology of the Cell, pp 681–726. 2nd ed. New York: Garland, 1989 |
|
Masiakowski P, Carroll RD: A novel family of cell surface receptors with tyrosine kinase-like domain. J Biol Chem 267: 26181– 26190, 1992 |
|
Litwack G: Biochemistry of hormones I: Peptide hormones. In Devlin TM (ed): Textbook of Biochemistry: With Clinical Correlations, p 847. New York: Wiley-Liss, 1992 |
|
Catt KJ, Dufau ML: Gonadotropic hormones: biosynthesis, secretion, receptors and actions. In Yen SSC, Jaffe RB (eds): Reproductive Endocrinology. 3rd ed. Philadelphia: WB Saunders, 1991 |
|
Jans DA: Nuclear signaling pathways for polypeptide ligands and their membrane receptors. FASEB J 8: 841– 847, 1994 |
|
Jans DA, Hassan G: Nuclear targeting by growth factors, cytokines, and their receptors: A role in signaling? Bioessays 20: 400– 411, 1998 |
|
Klinge CM, Rao ChV: The steroid hormone receptors. In Sciarra JJ (ed): Gynecology and Obstetrics, vol 5. Philadelphia: Harper & Row, 2000 |
|
McFarland KC, Sprengel R, Phillips HS et al: Lutropin-choriogonadotropin receptor: an unusual member of the G protein-coupled receptor family. Science 245: 494– 499, 1989 |
|
Loosfelt H, Misrahi M, Atger M et al: Cloning and sequencing of porcine LH-hCG receptor cDNA: Variants lacking transmembrane domain. Science 245: 525– 528, 1989 |
|
O'Dowd BR, Lefkowitz RJ, Caron MG: Structure of the adrenergic and related receptors. Annu Rev Neurosci 12: 67– 83, 1989 |
|
Baulieu EM: Cell membrane, a target for steroid hormones. Mol Cell Endocrinol 12: 247– 254, 1978 |
|
Pliam NB, Goldfine ID: High-affinity thyroid hormone binding sites on purified rat liver plasma membranes. Biochem Biophys Res Commun 79: 166, 1977 |
|
Richert N, Ryan RJ: Specific gonadotropin binding to Pseudomonas maltophilia. Proc Natl Acad Sci USA 74: 878– 882, 1977 |
|
Hamm HE: The many faces of G-protein signaling. J Biol Chem 273: 669– 672, 1998 |
|
Ji TH, Grossmann M, Ji I: G protein-coupled receptors. I. Diversity of receptor-ligand interactions. J Biol Chem 273: 17299– 17302, 1998 |
|
Simon MI, Strathmann MP, Gautam N: Diversity of G proteins in signal transduction. Science 252: 802– 808, 1991 |
|
Wang Z, Wang H, Ascoli M: Mutation of a highly conserved acidic residue present in the second intracellular loop of G protein-coupled receptors does not impair hormone binding or signal transduction of the luteinizing hormone/chorionic gonadotropin receptor. Mol Endocrinol 7: 85– 93, 1993 |
|
Gutkind JS: The pathways connecting G protein-coupled receptors to the nucleus through divergent mitogenactivated protein kinase cascades. J Biol Chem 273: 1839– 1842, 1998 |
|
Kaziro Y, Itoh H, Kozasa T, Nakafuku M, Satoh T: Structure and function of signal-transducing GTP-binding proteins. Annu Rev Biochem 60: 349– 400, 1991 |
|
Gether U, Kobilka BK: G protein-coupled receptors. II. Mechanism of agonist activation. J Biol Chem 273: 17979– 17982, 1998 |
|
Gilman AG: G proteins: Transducers of receptor-generated signals. Annu Rev Biochem 56: 615– 649, 1987 |
|
Spiegel AM, Shenker A, Weinstein LS: Receptor-effector coupling by G proteins: Implications for normal and abnormal signal transduction. Endocr Rev 13: 536– 565, 1992 |
|
Bourne HR: One molecular machine can transduce diverse signals. Nature 321: 814– 816, 1986 |
|
Birnbaumer L: Receptor-to-effector signaling through G proteins: Roles for beta gamma dimers as well as α subunits. Cell 71: 1069– 1072, 1992 |
|
Spiegel AM: Heterotrimeric GTP-binding proteins: an expanding family of signal transducers. Med Res Rev 12: 55– 71, 1992 |
|
Takai Y, Kaibuchi K, Kikuchi A, Kawata M: Small GTP-binding proteins. Int Rev Cytol 133: 187– 230, 1992 |
|
Pennington SR: GTP-binding proteins. 1: Heterotrimeric G proteins. Protein Profile 1: 169– 342, 1994 |
|
Berman DM, Gilman AG: Mammalian RGS proteins: Barbarians at the gate. J Biol Chem 273: 1269– 1272, 1998 |
|
Gutkind JS: Cell growth control by G protein-coupled receptors: From signal transduction to signal integration. Oncogene 17: 1331– 1342, 1998 |
|
Salesse R, Remy JJ, Levin JM et al: Towards understanding the glycoprotein hormone receptors. Biochimie 73: 109– 120, 1991 |
|
Segaloff DL, Sprengel R, Nikolics K, Ascoli M: The structure of the lutropin/choriogonadotropin receptor. Recent Prog Horm Res 46: 261– 303, 1990 |
|
Xie YB, Wang H, Segaloff DL: Extracellular domain of lutropin/choriogonadotropin receptor expressed in transfected cells binds choriogonadotropin with high affinity. J Biol Chem 265: 21411– 21414, 1990 |
|
Yarden Y, Ullrich A: Growth factor receptor tyrosine kinases. Annu Rev Biochem 57: 443– 478, 1988 |
|
Ullrich A, Coussens L, Hayflick JS et al: Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. Nature 309: 418– 425, 1984 |
|
Escobedo JA, Navankasatussas S, Cousens LS et al: A common PDGF receptor is activated by homodimeric A and B forms of PDGF. Science 240: 1532– 1534, 1988 |
|
Lee PL, Johnson DE, Cousens LS et al: Purification and complementary DNA cloning of a receptor for basic fibroblast growth factor. Science 245: 57– 60, 1989 |
|
White MF, Shoelson SE, Keutmann H, Kahn CR: A cascade of tyrosine phosphorylation in the 8-subunit activates the phosphotransferase activity of the insulin receptor. J Biol Chem 264: 2969– 2980, 1989 |
|
Rosen OM, Herrera R, Olowe Y et al: Phosphorylation activates the insulin receptor tyrosine protein kinase. Proc Natl Acad Sci USA 80: 3237– 3240, 1983 |
|
Cochet C, Gill GN, Meisenhelder J et al: C-kinase phosphorylates the epidermal growth factor receptor and reduces its epidermal growth factor-stimulated tyrosine protein kinase activity. J Biol Chem 259: 2553– 2558, 1984 |
|
Beguinot F, Smith RJ, Kahn CR et al: Phosphorylation of insulin-like growth factor I receptor by insulin receptor tyrosine kinase in intact cultured skeletal muscle cells. Biochemistry 27: 3222– 3228, 1988 |
|
White MF, Maron R, Kahn CR: Insulin rapidly stimulates tyrosine phosphorylation of a Mr 185,000 protein in intact cells. Nature 318: 183– 186, 1985 |
|
Low MG, Saltiel AR: Structural and functional roles of glycosyl-phosphatidyl-inositol in membranes. Science 239: 268– 275, 1988 |
|
Martelli AM, Gilmour RS, Bertagnolo V et al: Nuclear localization and signaling activity of phosphoinositidase C beta in Swiss 3T3 cells. Nature 358: 242– 245, 1992 |
|
Fuh G, Mulkerrin MG, Bass S et al: The human growth hormone receptor. J Biol Chem 265: 3111– 3115, 1990 |
|
Boutin J-M, Jolicoeur C, Okamura H et al: Cloning and expression of the rat prolactin receptor, a member of the growth hormone/prolactin receptor gene family. Cell 53: 69– 72, 1988 |
|
Carter-Su C, Stubbart JR, Wang XY et al: Phosphorylation of highly purified growth hormone receptors by a growth hormone receptor-associated tyrosine kinase. J Biol Chem 264: 18654– 18661, 1989 |
|
Buckley AR, Crowe PD, Russell DH: Rapid activation of protein kinase C in isolated rat liver nuclei by prolactin, a known hepatic mitogen. Proc Natl Acad Sci USA 85: 8649– 8653, 1988 |
|
Hille B: Ionic Channels of Excitable Membranes. Sunderland MA: Sinauer, 1984 |
|
Changeux JP: The acetylcholine receptor: Its molecular biology and biotechnological prospects. Bioessays 10: 48– 54, 1989 |
|
Huganir RL: Phosphorylation of purified ion channel proteins. In Kaezmarek LK, Levitan IB (eds): Neuromodulation: The Biochemical Control of Neuronal Excitability, pp 86–99. New York: Oxford University Press, 1987 |
|
Huganir RL, Delcour AH, Greengard P, Hess GP: Phosphorylation of the nicotinic acetylcholine receptor regulates its rate of desensitization. Nature 321: 774– 776, 1986 |
|
Birnbaumer L, Codina J, Yatani A et al: Molecular basis of regulation of ionic channels by G proteins. Recent Prog Horm Res 45: 121– 206, 1989 |
|
Levitan IB: Modulation of ion channels in neurons and other cells. Annu Rev Neurosci 11: 119– 136, 1988 |
|
Schulz S, Chinkers M, Garbers DL: The guanylate cyclase/receptor family of proteins. FASEB J 3: 2026– 2035, 1989 |
|
Hokin LE: Receptors and phosphoinositide-generated second messengers. Annu Rev Biochem 54: 205– 235, 1985 |
|
Rhee SG, Choi KD: Regulation of inositol phospholipid-specific phospholipase C isozymes. J Biol Chem 267: 12393– 12396, 1992 |
|
Wu D, Lee CH, Rhee SG, Simon MI: Activation of phospholipase C by the subunits of the Gq and G11 proteins in transfected Cos-7 cells. J Biol Chem 267: 1811– 1817, 1992 |
|
Katz A, Wu D, Simon MI: Subunits beta gamma of heterotrimeric G protein activate 82 isoform of phospholipase C. Nature 360: 686– 689, 1992 |
|
Berridge MJ, Irvine RF: Inositol phosphates and cell signaling. Nature 341: 197– 205, 1989 |
|
Nishizuka Y: Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science 258: 607– 614, 1992 |
|
Thompson EB: Single receptors, dual second messengers [Comment]. Mol Endocrinol 6: 501, 1992 |
|
Gudermann T, Nichols C, Levy FO et al: Ca2+ mobilization by the LH receptor expressed in Xenopus oocytes independent of 3',5'-cyclic adenosine monophosphate formation: Evidence for parallel activation of two signaling pathways. Mol Endocrinol 6: 272– 278, 1992 |
|
Naor Z, Childs GV: Binding and activation of gonadotropin releasing hormone receptors in pituitary and gonadal cells. Int Rev Cytol 103: 147– 187, 1986 |
|
Cotecchia S, Kobilka BK, Daniel KW et al: Multiple second messenger pathways of α-adrenergic receptor subtypes expressed in eukaryotic cells. J Biol Chem 265: 63– 69, 1990 |
|
Kenakin T: Are receptors promiscuous? Intrinsic efficacy as a transduction phenomenon. Life Sci 43: 1095– 1101, 1988 |
|
Ignar-Trowbridge DM, Nelson KG, Bidwell MC et al: Coupling of dual signaling pathways: Epidermal growth factor action involves the estrogen receptor. Proc Natl Acad Sci USA 89: 4658– 4662, 1992 |
|
Curtis SW, Washburn T, Sewall C et al: Physiological coupling of growth factor and steroid receptor signaling pathways: Estrogen receptor knockout mice lack estrogen-like response to epidermal growth factor. Proc Natl Acad Sci USA 93: 12626– 12630, 1996 |
|
Smith CL: Cross-talk between peptide growth factor and estrogen receptor signaling pathways. Biol Reprod 58: 627– 632, 1998 |
|
El-Tanani MKK, Green CD: Interaction between estradiol and growth factors in the regulation of specific gene expression in MCF-7 human breast cancer cells. J Steroid Biochem Mol Biol 60: 269– 276, 1997 |
|
El-Tanani MKK, Green CD: Two separate mechanisms for ligand-independent activation of the estrogen receptor. Mol Endocrinol 11: 928– 937, 1997 |
|
Power RF, Lydon JP, Conneely OM, O'Malley BW: Dopamine activation of an orphan of the steroid receptor superfamily. Science 252: 1546– 1548, 1991 |
|
Power RF, Mani SK, Codina J et al: Dopaminergic and ligand-independent activation of steroid hormone receptors. Science 254: 1636– 1639, 1991 |
|
Shi QJ, Lei ZM, Rao ChV, Lin J: Novel role of human chorionic gonadotropin in differentiation of human cytotrophoblasts. Endocrinology, 132:1387–1395, 1993 |
|
Licht P, Lei ZM, Rao ChV, Merz WM: The presence and functional relevance of hCG/LH receptors in normal human placentae and choriocarcinomas. In Barnea ER, Check JH, Grudzinskas JG, Maruo T (eds): Implantation and Early Pregnancy in Humans, pp 234–246. Pearl River, NY: Parthenon Publishing Group, 1994 |
|
Licht P, Lei ZM, Rao ChV, Merz WM: A novel effect of bredfeldin A (BFA): inhibition of hCG-&b.alpha; and &b.beta;-subunit mRNA levels in choriocarcinoma cells and evidence for a possible intracrine regulation of hCG biosynthesis [Abstract 1555]. Proceedings of the Endocrine Society Annual Meeting, San Antonio, Texas, 1992 |
|
Khan-Dawood FS, Dawood MY, Satyaswaroop PG: Baboon corpus luteum: Immunohistochemical localization of progesterone receptors [Abstract P348]. Annual meeting of the Society for Gynecologic Investigation, Toronto, Ontario, 1993 |
|
Goldfine ID, Smith GJ: Binding of insulin to isolated nuclei. Proc Natl Acad Sci USA 73: 1427– 1431, 1976 |
|
Rao ChV, Mitra S: Subcellular distribution of prostaglandin and gonadotropin receptors in bovine corpora lutea. Biochem Biophys Res Commun 76:636–643, 1977 |
|
Mitra S, Rao ChV: Receptors for gonadotropins and prostaglandins in lysosomes of bovine corpora lutea. Arch Biochem Biophys 185:126–133, 1978 |
|
Mitra S, Rao ChV: Gonadotropin and prostaglandin binding sites in rough endoplasmic reticulum and Golgi fractions of bovine corpora lutea. Arch Biochem Biophys 191:331–340, 1978 |
|
Rao ChV, Mitra S: Gonadotropin and prostaglandins binding sites in nuclei of bovine corpora lutea. Biochim Biophys Acta 584:454–466, 1979 |
|
Yankner BA, Shooter EM: Nerve growth factor in the nucleus: Interaction with receptors on the nuclear membrane. Proc Natl Acad Sci USA 76: 1269– 1273, 1979 |
|
Rao ChV, Mitra S, Carman FR Jr: Characterization of gonadotropin binding sites in the intracellular organelles of bovine corpora lutea and comparison with those in plasma membranes. J Biol Chem 256:2628–2634. 1981 |
|
Rao ChV, Mitra S, Sanfilippo J, Carman FR Jr: The presence of gonadotropin binding sites in the intracellular organelles of human ovaries. Am J Obstet Gynecol 139:655–660, 1981 |
|
Schumm DE, Webb TE: Insulin-modulated transport of RNA from isolated liver nuclei. Arch Biochem Biophys 210: 275– 279, 1981 |
|
Purrello F, Vigneri R, Clawson GA, Goldfine ID: Insulin stimulation of nucleoside triphosphatase activity in isolated nuclear envelopes. Science 216: 1005– 1007, 1982 |
|
Rao ChV, Fields MJ, Chen TT et al: Changes in gonadotropin binding sites in intracellular organelles and plasma membrane during luteal growth, development and regression. Exp Cell Res 144:285–295, 1983 |
|
Chegini N, Rao ChV, Carman FR Jr: Internalization of 125I-human choriogonadotropin in luteal slices: A biochemical study. Exp Cell Res 151:466–482, 1984 |
|
Chegini N, Rao ChV, Cobbs G: A quantitative electron microscope autoradiographic study on 125I-human choriogonadotropin internalization in bovine luteal slices. Exp Cell Res 151:483–493, 1984 |
|
Chegini N, Rao ChV, Cobbs G: A quantitative electron microscope autoradiographic study on 3H-prostaglandin E1and F2α internalization in bovine luteal slices. Mol Cell Endocrinol 38:117–129, 1984 |
|
Kushnaryov VM, MacDonald HS, Sedmak JJ, Grossberg SE: Murine interferon-&b.beta; receptor-mediated endocytosis and nuclear membrane binding. Proc Natl Acad Sci USA 82: 3281– 3285, 1985 |
|
MacDonald HS, Kushnaryov VM, Sedmak JJ, Grossberg SE: Transport of &b.gamma;-interferon into the cell nucleus may be mediated by nuclear membrane receptors. Biochem Biophys Res Commun 138: 254– 260, 1986 |
|
Ramani N, Chegini N, Rao ChV et al: The presence of epidermal growth factor binding sites in the intracellular organelles of term human placenta. J Cell Sci 84:19–40, 1986 |
|
Chegini N, Rao ChV: Quantitative electron microscope autoradiographic studies on 125I-epidermal growth factor internalization in term human placenta. J Cell Sci 84:41–52, 1986 |
|
Ramani N, Rao ChV: Direct stimulation of nucleoside triphosphatase activity in bovine luteal nuclear membranes by human chorionic gonadotropin. Endocrinology 120:2468–2473, 1987 |
|
Toledo A, Ramani N, Rao ChV: Direct stimulation of nucleoside triphosphatase activity in human ovarian nuclear membranes by human chorionic gonadotropin. J Clin Endocrinol Metab 65:305–309, 1987 |
|
Bouche G, Gas N, Prats H et al: Basic fibroblast growth factor enters the nucleolus and stimulates the transcription of ribosomal genes in ABAE cells undergoing G0 → G1 transition. Proc Natl Acad Sci USA 84: 6770– 6774, 1987 |
|
Podlecki DA, Smith RM, Kao M et al: Nuclear translocation of the insulin receptor. A possible mediator of insulin's long-term effects. J Biol Chem 262: 3362– 3368, 1987 |
|
Qwarnstrom EE, Page RC, Gillis S, Dower SK: Binding, internalization, and intracellular localization of interleukin-1&b.beta; in human diploid fibroblasts. J Biol Chem 263: 8261– 8269, 1988 |
|
Miller DS: Stimulation of RNA and protein synthesis by intracellular insulin. Science 240: 506– 509, 1988 |
|
Oechsli M, Rao ChV, Chegini N: Human chorionic gonadotropin increases chromatin solubility in isolated bovine and human luteal nuclei. Biol Reprod 41:753–760, 1989 |
|
Hocher B, Rubens C, Henson J et al: Intracellular distribution of endothelin-1 receptors in rat liver cells. Biochem Biophys Res Commun 184: 498– 503, 1992 |
|
Buckley AR, Montgomery DW, Hendrix MJC et al: Identification of prolactin receptors in hepatic nuclei. Arch Biochem Biophys 296: 198– 206, 1992 |
|
Weitzmann MN, Savage N: Nuclear internalization and DNA binding activities of interleukin-1, interleukin-1 receptor and interleukin-1/receptor complexes. Biochem Biophys Res Commun 187: 1166– 1171, 1992 |
|
Nakanishi Y, Kihara K, Mizuno K et al: Direct effect of basic fibroblast growth factor on gene transcription in a cell-free system. Proc Natl Acad Sci USA 89: 5216– 5220, 1992 |
|
Panagakos FS, Kumar S: Nuclear localization of tumor necrosis factor-alpha in human osteoblast-like cells. Biochem Biophys Res Commun 201: 1445– 1450, 1994 |
|
Holt SJ, Alexander P, Inman CB, Davies DE: Epidermal growth factor induced tyrosine phosphorylation of nuclear proteins associated with translocation of epidermal growth factor receptor into the nucleus. Biochem Pharmacol 47: 117– 126, 1994 |
|
Lobie PE, Wood TJ, Chen CM et al: Nuclear translocation and anchorage of the growth hormone receptor. J Biol Chem 269: 31735– 31746, 1994 |
|
Jimenez E, Vinson GP, Montiel M: Angiotensin II (AII)-binding sites in nuclei from rat liver: Partial characterization of the mechanism of All accumulation in nuclei. J Endocrinol 143: 449– 453, 1994 |
|
Cao H, Lei ZM, Bian L, Rao ChV: Functional nuclear epidermal growth factor receptors in human choriocarcinoma JEG-3 cells and normal human placenta. Endocrinology 136:3163–3172, 1995 |
|
Shah N, Zhang S, Harada S et al: Electron microscopic visualization of insulin translocation into the cytoplasm and nuclei of intact H35 hepatoma cells using covalently linked nanogold-insulin. Endocrinology 136: 2825– 2835, 1995 |
|
Holt SJ, Alexander P, Inman CB, Davies DE: Ligand-induced translocation of epidermal growth factor receptor to the nucleus of NR6/HER fibroblasts is serum dependent. Exp Cell Res 217: 554– 558, 1995 |
|
Lin YZ, Yao SY, Hawiger J: Role of the nuclear localization sequence in fibroblast growth factor-1-stimulated mitogenic pathways. J Biol Chem 271: 5305– 5308, 1996 |
|
Stachowiak MK, Maher PA, Joy A et al: Nuclear localization of functional FGF receptor 1 in human astrocytes suggests a novel mechanism for growth factor action. Mol Brain Res 38: 161– 165, 1996 |
|
Stachowiak MK, Maher PA, Joy A et al: Nuclear accumulation of fibroblast growth factor receptors is regulated by multiple signals in adrenal medullary cells. Mol Biol Cell 7: 1299– 1317, 1996 |
|
Maher PA: Identification and characterization of a novel, intracellular isoform of fibroblast growth factor receptor-1 (FGFR-1). J Cell Physiol 169: 380– 390, 1996 |
|
Li W, Fawcett J, Widmer HR et al: Nuclear transport of insulin-like growth factor-I and insulin-like growth factor binding protein-3 in opossum kidney cells. Endocrinology 138: 1763– 1766, 1997 |
|
Stachowiak EK, Maher PA, Tucholski J et al: Nuclear accumulation of fibroblast growth factor receptors in human glial cells—association with cell proliferation. Oncogene 14: 2201– 2211, 1997 |
|
Bian L, Lei Z, Rao ChV: Mitogen-activated protein kinase is involved in epidermal-growth-factor-regulated protein phosphorylation in nuclear membranes isolated from JEG-3 human choriocarcinoma cells. Eur J Biochem 253:545–551, 1998 |
|
Bhattacharya M, Peri KG, Almazan G et al: Nuclear localization of prostaglandin E2 receptors. Proc Natl Acad Sci USA 95: 15792– 15797, 1998 |
|
Pederson T: Growth factors in the nucleolus? J Cell Biol 143: 279– 281, 1998 |
|
Arese M, Chen Y, Florkiewicz RZ et al: Nuclear activities of basic fibroblast growth factor: potentiation of low-serum growth mediated by natural or chimeric nuclear localization signals. Mol Biol Cell 10: 1429– 1444, 1999 |
|
Pearse BMF, Bretscher MS: Membrane recycling by coated vesicles. Annu Rev Biochem 50: 85, 1981 |
|
Kahn CR, Baird KL, Jarrett DB, Flier JS: Direct demonstration that receptor cross-linking or aggregation is important in insulin action. Proc Natl Acad Sci USA 75: 4209– 4213, 1978 |
|
Ascoli M: Lysosomal accumulation of the hormonereceptor complex during receptor-mediated endocytosis of human choriogonadotropin. J Cell Biol 99: 1242– 1250, 1984 |
|
Pastan IH, Willingham MC: Receptor-mediated endocytosis of hormones in cultured cells. Annu Rev Physiol 43: 239– 250, 1981 |
|
Marshall S: Kinetics of insulin receptor internalization and recycling in adipocytes. J Biol Chem 260: 4136– 4144, 1985 |
|
Ascoli M, Segaloff DL: On the fates of receptor-bound ovine luteinizing hormone and human chorionic gonadotropin in cultured Leydig tumor cells: Demonstration of similar rates of internalization. Endocrinology 120: 1161– 1172, 1987 |
|
Cooke BA, Platts EA, Abayasekera DRE, Rose MP: Mechanisms of hormone-induced desensitization of adenylate cyclase. Biochem Soc Trans 17: 633– 635, 1989 |
|
Menon KMJ, Nair AK, Wang L, Peegel H: Regulation of luteinizing hormone receptor mRNA expression by a specific RNA binding protein in the ovary. Mol Cell Endocrinol 260: 109-116, 2007 |
|
Ascoli M: Internalization and degradation of receptor-bound human choriogonadotropin in Leydig tumor cells. J Biol Chem 257: 13306– 13311, 1982. |
|
Hirota K, Hirota T, Aguilera G, Catt KJ: Hormone induced redistribution of calcium activated phospholipid dependent protein kinase in pituitary gonadotrophs. J Biol Chem 260: 3243– 3246, 1985 |
|
Naor Z, Zer J, Zakut H, Hermon J: Characterization of pituitary calcium-activated phospholipid-dependent protein kinase: Redistribution by gonadotropin releasing hormone. Proc Natl Acad Sci USA 82: 8203– 8207, 1985 |
|
McArdle CA, Conn PM: Hormone stimulated redistribution of gonadotrope protein kinase C in vivo: Dependence on calcium mobilization. Mol Pharmacol 29: 570– 576, 1986 |
|
Posner BI, Kelley PA, Friesen HG: Prolactin receptor in rat liver: possible induction by prolactin. Science 188: 57– 59, 1978 |
|
Nimrod A, Tsafriri A, Linder HR: In vitro induction of binding sites for HCG in rat granulosa cells by FSH. Nature 267: 632– 633, 1977 |
|
Williams LT, Lefkowitz RJ, Watanabe AM et al: Thyroid hormone regulation of beta-adrenergic receptor number. J Biol Chem 252: 2787– 2789, 1977 |
|
Lekstrom-Himes J, Xanthopoulos KG: Biological role of the CCAAT/enhancer-binding protein family of transcription factors. J Biol Chem 273: 28545– 28548, 1998 |
|
Kwok RP, Lundblad JR, Chrivia JC et al: Nuclear protein CBP is a coactivator for the transcription factor CREB. Nature 370: 223– 226, 1994 |
|
Chakravarti D, LaMorte VJ, Nelson MC et al: Role of CBP/P300 in nuclear receptor signaling. Nature 383: 99– 103, 1996 |
|
Kamei Y, Xu L, Heinzel T et al: A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell 85: 403– 414, 1996 |
|
Habener JF: Cyclic AMP response element binding proteins: A cornucopia of transcription factors. Mol Endocr 4: 1087– 1094, 1990 |
|
Johnson PF, McKnight SL: Eukaryotic transcriptional regulatory proteins. Annu Rev Biochem 58: 799– 839, 1989 |
|
Evans RM: The steroid and thyroid hormone receptor superfamily. Science 240: 889– 895, 1988 |
|
Stanley FM: An element in the prolactin promoter mediates the stimulatory effect of insulin on transcription of the prolactin gene. J Biol Chem 267: 16719– 16726, 1992 |
|
Choi BH, Park CJ, Rho HM: Insulin activates the hepatitis B virus X gene through the activating protein-1 binding site in HepG2 cells. DNA Cell Biol 17: 951– 956, 1998 |
|
Hasegawa J, Osatomi K, Wu RF, Uyeda K: A novel factor binding to the glucose response elements of liver pyruvate kinase and fatty acid synthase genes. J Biol Chem 274: 1100– 1107, 1999 |
|
Zelzer E, Levy Y, Kahana C et al: Insulin induces transcription of target genes through the hypoxia-inducible factor HIF-1alpha/ARNT. EMBO J 17: 5085– 5094, 1998 |
|
Darnell JEJ: STATs and gene regulation. Science 277: 1630– 1635, 1997 |
|
Liu X, Robinson GW, Wagner KU et al: Stat5 is mandatory for adult mammary gland development and lactogenesis. Genes Dev 11: 179– 186, 1997 |
|
Udy GB, Towers RP, Snell RG et al: Requirement of STAT5b for sexual dimorphism of body growth rates and liver gene expression. Proc Natl Acad Sci USA 94: 7239– 7244, 1997 |
|
Reaven GM: Insulin-independent diabetes mellitus: Metabolic characteristics. Metabolism 29: 445– 454, 1980 |
|
Farfel Z, Brickman AS, Kaslow HR et al: Defect of receptor-cyclase coupling protein in pseudohypoparathyroidism. N Engl J Med 303: 237– 242, 1980 |
|
Wilson K, Eisenbarth GS: Immunopathogenesis and immunotherapy of type I diabetes. Annu Rev Med 41: 497– 508, 1990 |
|
Rees Smith B, Hall R: Thyroid-stimulating immunoglobulins in Graves' disease. Lancet 2: 427– 430, 1974 |
|
Mehdi SQ, Nussey SS: A radioligand receptor assay for the long-acting thyroid stimulator. Biochem J 145: 105– 111, 1975 |
|
Margolis B, Rhee S, Felder S et al: EGF induces tyrosine phosphorylation of phospholipase C-II: A potential mechanism for EGF receptor signaling. Cell 57: 1101– 1107, 1989 |
|
McCormick F: Ras GTPase activating protein: Signal transmitter and signal terminator. Cell 56: 5– 8, 1989 |
|
Burgoyne RD: Small GTP-binding proteins. Trends Biochem Sci 14: 394– 396, 1989 |
|
Kouzarides T, Ziff E: Leucine zippers of fos, jun, and GCN4 dictate dimerization specificity and thereby control DNA binding. Nature 340: 568– 571, 1989 |
|
DePablo F, Eastman RC, Roth J, Gorden P: Plasma prolactin in acromegaly before and after treatment. J Clin Endocrinol Metab 53: 344– 352, 1981 |
|
Mann K, Schneider N, Hoermann R: Thyrotropic activity of acidic isoelectric variants of human chorionic gonadotropin from trophoblastic tumors. Endocrinology 118: 1558– 1566, 1986 |
|
Dhanasekaran N, Heasley LE, Johnson GL: G protein-coupled receptor systems involved in cell growth and oncogenesis. Endocr Rev 16: 259– 270, 1995 |
|
Arvanitakis L, Geras-Raaka E, Varma A et al: Human herpesvirus KSHV encodes a constitutively active G protein-coupled receptor linked to cell proliferation. Nature 385: 347– 350, 1997 |
|
Layman LC: Mutations in human gonadotropin genes and their physiologic significance in puberty and reproduction. Fertil Steril 71: 201– 218, 1999 |
|
Shenker A, Laue L, Kosugi S et al: A constitutively activating mutation of the luteinizing hormone receptor in familial male precocious puberty. Nature 365: 652– 654, 1993 |
|
Sultan C, Lumbroso S: LH receptor defects. In Kempers RD, Cohen J, Haney AF, Younger JB (eds): Fertility and Reproductive Medicine, pp 769–782. New York: Elsevier Science, 1998 |
|
Aittomaki K, Lucena JL, Pakarinen P et al: Mutation in the follicle-stimulating hormone receptor gene causes hereditary hypergonadotropic ovarian failure. Cell 82: 959– 968, 1995 |
|
Tapanainen JS, Aittomaki K, Min J et al: Men homozygous for an inactivating mutation of the follicle-stimulating hormone (FSH) receptor gene present variable suppression of spermatogenesis and fertility. Nat Genet 15: 205– 206, 1997 |
|
Tapanainen JS, Vaskivuo T, Aittomaki K, Huhtaniemi IT: Inactivating FSH receptor mutations and gonadal dysfunction. Mol Cell Endocrinol 145: 129– 135, 1998 |
|
Dierich A, Sairam MR, Monaco L et al: Impairing follicle-stimulating hormone (FSH) signaling in vivo: Targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc Natl Acad Sci USA 95: 13612– 13617, 1998 |
|
Rodway MR, Rao ChV: A novel perspective on the role of human chorionic gonadotropin during pregnancy and in gestational trophoblastic disease. Early Pregnancy Biol Med 1:176–187, 1995 |
|
Rao ChV: The beginning of a new era in reproductive biology and medicine: Expression of low levels of functional luteinizing hormone/human chorionic gonadotropin receptors in nongonadal tissues. J Physiol Pharmacol 47(Suppl 1):41–53, 1996 |
|
Rao ChV, Sanfilippo JS: New understanding in the biochemistry of implantation: Potential direct roles of luteinizing hormone and chorionic gonadotropin. Endocrinologist 7:107–111, 1997 |
|
Rao ChV: Potential novel roles of luteinizing hormone and human chorionic gonadotropin during early pregnancy in women. Early Pregnancy Biol Med 3:1–9, 1997 |
|
Rao ChV: A paradigm shift on the targets of luteinizing hormone/human chorionic gonadotropin actions in the body. J Bellevue Obstet Gynecol Soc 15:26–32, 1999 |
|
Rao ChV: Novel concepts in neuroendocrine regulation of reproductive tract functions. In: Bazer FW (ed), Endocrinology of Pregnancy, pp 125–144. Totowa, NJ: The Human Press, 1998 |
|
Lukacs H, Hiatt ES, Lei ZM, Rao ChV: Peripheral and intracerebroventricular administration of human chorionic gonadotropin alters several hippocampus-associated behaviors in cycling female rats. Horm Behav 29:42–58, 1995 |
|
Toth P, Lukacs H, Hiatt ES et al: Administration of human chorionic gonadotropin affects sleep-wake phases and other associated behaviors in cycling female rats. Brain Res 654: 181– 190, 1994 |
|
Scatchard G: The attraction of proteins for small molecules and ions. Ann NY Acad Sci 51: 660– 672, 1949 |